MARCH 2023 I Volume 44, Issue 1
Foundational Aerothermodynamic and Ablative Models in Hypersonic Flight Environments
MARCH 2023
Volume 44 I Issue 1
IN THIS JOURNAL:
- Issue at a Glance
- Chairman’s Message
Workforce of the Future
- Foundational Aerothermodynamic and Ablative Models in Hypersonic Flight Environments
Technical Articles
- Test and Evaluation as a Continuum
- Operational Test and Evaluation (OT&E) for Rapid Software Development
- Bayesian Methods in Test and Evaluation
News
- Association News
- Chapter News
- Corporate Member News
Workforce of the Future:
Foundational Aerothermodynamic and Ablative Models in Hypersonic Flight Environments
- DEVCOM Army Research Laboratory, Aberdeen Proving Ground, MD 21005, United States
- Applied Research Laboratory, The Pennsylvania State University, University Park, PA 16802, United States
Abstract
Aero-propulsion, in particular applications involving hypersonic flight, benefits from ultra-high temperature ceramics (UHTC) efficiency. Atomistic simulations were carried out to better understand the thermomechanical characteristics of these materials at the elevated temperatures and pressures of hypersonic flight. To determine the first oxidation of α-SiC (silicon carbide), both the classical molecular dynamics simulation code LAMMPS (Large-scale Atomic/Molecular Massively Parallel Simulator) and the reactive ReaxFF potential were used. The ablation of SiO3, CO, CO2, C2O2, and C2O3 molecules in these simulations provide evidence of the first ablative characteristics of α-SiC as well as the distinctive parabolic reaction kinetics found in very harsh settings. The temperature range selected for these simulations was 26 °C to 2,000 °C. Due to the unique oxidation kinetics present in these extreme environments, further research into the oxidation of UHTC would require the use of newly parameterized reactive potentials. This work assesses the effect of extreme environments and the volatilization of molecules from polycrystalline surfaces as a result of the unique oxidation kinetics that are present in these extreme environments.
Keywords: Hypersonic Flight; Ultra-High Temperature Ceramics; Atomistic Simulation; Molecular Dynamics Simulation
Introduction
As a result of their high melting temperatures, high thermal conductivities, low thermal expansion, and strong oxidation resistance, ultra-high temperature ceramics (UHTC) are materials of considerable interest in the area of aero-propulsion.1–4 These qualities also make them promising materials for hypersonic flight applications. Transition metal carbides and borides are well-suited for use as leading edge materials, which will be necessary to increase efficiency and stability of aircraft in the future.3 These material qualities can be further improved by integrating SiC into composites to make ultra-high temperature ceramic matrix composites (UHTCMC)2,3,5,6.
The chemical reactions that occur at the surfaces of UHTCMC have parabolic kinetics at high temperatures, and these reactions are generally distinct from those that take place at low temperatures.3,7 The systems in question are mostly unreactive at their typical operating temperatures (e.g., below 1,500 °C to 1,800 °C). However, at high temperatures, a tremendous amount of thermal energy is applied to the systems which reduces the energy barriers for unfavorable reaction kinetics.4,5,7 The propensity of these materials to oxidize at necessary high operating temperatures is regarded as the primary impediment in the research and development of these materials for aero-propulsion, and is the primary contributor to the lack of progress in developing new materials for these applications. This phenomenon is particularly common in composite systems which incorporate both ionic and covalent bonds, or if the melting points of the various components in the system vary much from one another. It is hypothesized that the physical chemistry at the oxidation site and the transport of the reactants to this site are crucial to the oxidation behavior of these materials.1,6,7 Furthermore, this project’s goal is to create an atomistic ablative model to better comprehend the thermal and mechanical features of the UHTCMC environment. This will be done by studying the causes of SiC ablation failure under harsh conditions of hypersonic flight with the goal of improving performance and extending UHTC lifespan.
Materials & Methods
Classical molecular dynamics (MD) simulations were performed to determine the thermomechanical and oxidation properties of the UHTC. These atomic simulations were run using the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) code developed at Sandia National Laboratory.8 Newton’s equation of motion was used in the MD simulations to track the atomic-level dynamics of a system through time. In order to carry out and comprehend the oxidation processes of the UHTC using MD, the interatomic potential used must allow for charge transfer and the formation of new species; thus, a reactive interatomic potential is required.9 To accomplish this, the ReaxFF interatomic potential and charge equilibration, already implemented in the LAMMPS source code, were used. The ReaxFF potential energy of the system is depicted in the following simplified equation of the atoms with physical bonds, non-bonded interacting atoms, and the charge transfer scheme.9,10
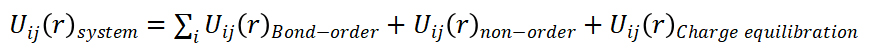
Polycrystalline SiC surfaces were exposed to oxidation at high temperatures and pressures in these MD simulations. From these simulations, the initial molecular species that ablate from the oxidized polycrystalline SiC surface were depicted. The temperature of hypersonic environments in the MD simulations was controlled with a Langevin thermostat with a timestep of 0.25 femtoseconds.11,12 Using these simulations, the initial oxidation and ablation behavior for the deposition of molecular and atomic oxygen onto the surface of the polycrystalline SiC was predicted. In the model, the system was brought from 3 Bar to 30 Bar as the simulation environment was heated from 26 C to 1726 C. Atomic and molecular oxygen were then added to the simulation with an initial velocity of Mach 10 which was quickly decreased to Mach 0.1 by the shock boundary layer where oxidation of the SiC surface occurs. If the oxidized species formed moves to the top of the simulation box, the atoms are deleted from the simulation to represent species being ablated from the surface. The atoms at the bottom of the simulation box are kept fixed while the rest of the atoms are under a thermostat (Figure 1). In these simulations, the rates of oxidation of different SiC grain surfaces and grain boundaries can be predicted, which will help reduce the number of experimental oxidation tests needed. Polyhedral Template Mapping was used in conjunction with coordination analysis to systematically identify hexagonal bulk crystal structures from grain boundaries and defective areas (Figure 1). Visualization for the MD simulations was done using the OVITO code.13
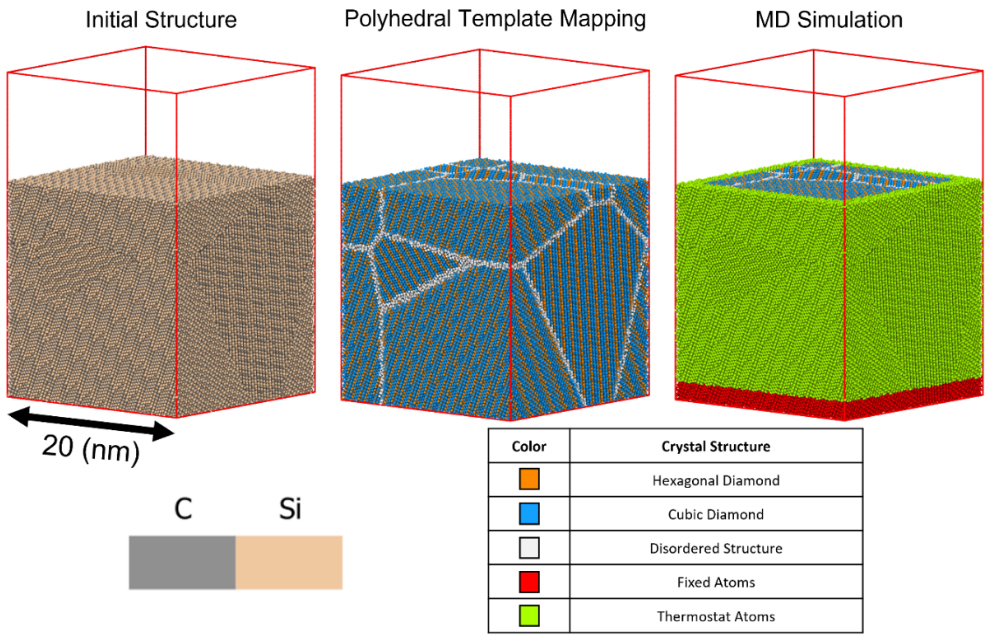
Figure 1 – Visual image of the initial MD simulation for the polycrystalline SiC, with color coordinated atoms. Polyhedral template mapping was used to depict the individual grains and grain boundaries. The rightmost image depicts the initial MD simulation build and indicates which atoms were thermostat and which were kept fixed during the simulation to stabilize the potential energy of the system.
Results
Through classical MD simulations, the oxidization of α-SiC was observed for polycrystalline structures. An illustration of the initial oxidization of the α-SiC is shown in Figure 2. As oxygen atoms collide with the α-SiC surface in the shock boundary layer of the hypersonic environments, a new active oxide film is formed. The oxidation of polycrystalline α-SiC may be broken down into three stages, first the oxidation of the unordered grain boundary α-SiC surfaces, then the oxidation of the surface terminations, and lastly oxygen diffusion through the SiO2 layer occurs. Segregation of SiO and C rich regions was observed in the oxide layer. There was also an increase in the oxide layer thickness on the α-SiC surface as the simulation pressure and temperature increased.
These simulations allowed us to predict the ablation behavior of individual molecules of SiO3, CO, CO2, C2O2, and C2O3 as they evaporated from the surface of the polycrystalline a-SiC (Figures 3 and 4). These simulations will allow for future in-depth examination of UHTC oxidation behaviors to better comprehend the peculiar parabolic reaction kinetics that occur in such severe settings.2,3,7
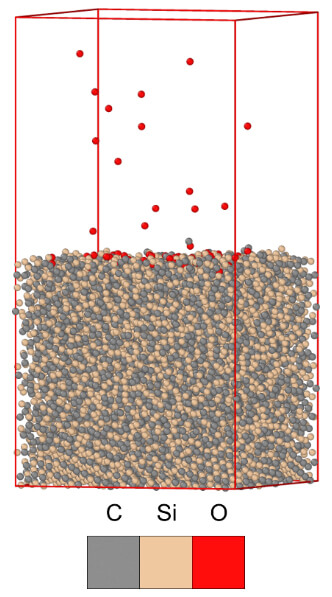
Figure 2 – MD simulation with the initial atomic oxidation of the hypersonic environment.
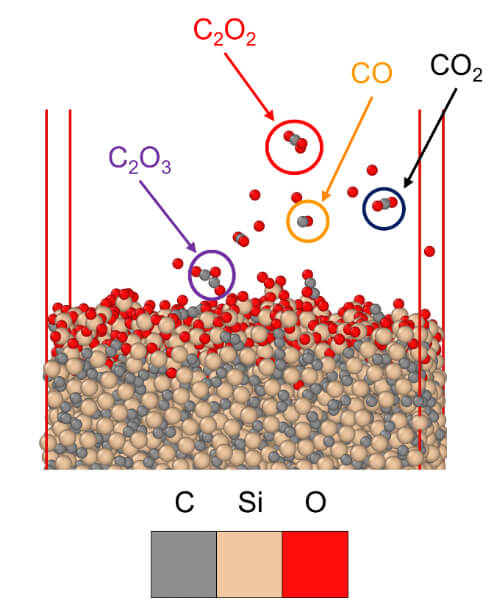
Figure 3 – Visual depiction of the ablation of CO, CO2, C2O2, and C2O3 molecules during the oxidation of polycrystalline a-SiC. A pressure of 30 Bar and a temperature of 1726 °C were used in the simulation.
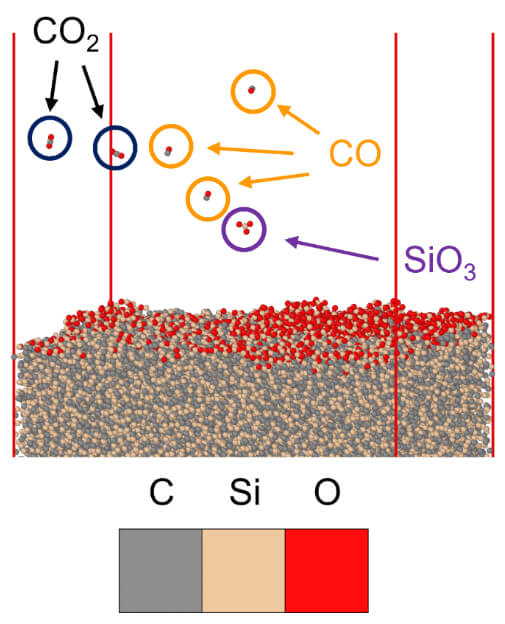
Figure 4 – Visual depiction of the ablation of SiO3, CO, CO2, and C2O2 molecules during the oxidation of polycrystalline a-SiC. A pressure of 30 Bar and a temperature of 1526 °C were used in the simulation.
Conclusions
The purpose of the next generation of high temperature propulsion material systems is to provide our military with strong overmatch capability for operation in all extreme environmental conditions. In this work, the ReaxFF interatomic potential was used to develop a fundamental kinetic model to understand the atomistic ablation of UHTC in hypersonic conditions. Using structural characterization and kinetic modelling, we predict the production of a silicon oxide layer on the surface of α-SiC and its structure in hypersonic environments. The ablation of SiO3, CO, CO2, C2O2, and C2O3 molecules from the α-SiC surface is also predicted at different temperatures.
Further investigation of traditional UHTC compositions is needed to better understand their thermomechanical properties regarding oxidization resistance in extreme environments. Next steps include the oxidation of additional transition metal carbides and borides as the current interatomic potentials developed for other possible UHTC are non reactive, which does not allow for charge transfer as is required for oxidation simulations. The ReaxFF interatomic potential must be parameterized for the remaining components in the target systems before oxide simulations can be run. To verify the new interatomic potentials for the new systems, density functional theory (DFT) computations must be performed. These calculations will be used to determine defect energies, formation energies, the equations of state for volume relaxations, adsorption energies, and the mechanical properties of the individual and composite systems. This will allow researchers to examine the effects of chemical bonding, defect density, composition, microstruture, and other factors on the oxidation behaviors of UHTC and UHTCMC.
Acknowledgements
The authors acknowledge financial support from the US Army Research Laboratory, the DOD High-Performance Computing Modernization Program (HPCMP), and the HPC Internship Program (HIP). The computations were run on the Narwhal supercomputer, which was kindly provided by the Department of Navy Research and Development Center. Dr. Slapikas would like to thank his advisors at the Army Research Laboratory, especially Drs. Ghoshal, Bravo, Murugan, and McGowan, for helping learn and grow professionally and academically during this project. He would also like to give thanks to Dr. Douglas Wolfe, his Ph.D. adviser at Penn State, for helping refine the simulations and his mentorship in the laboratory.
References
(1) DeGregoria, A. Creep and Oxidation of Hafnium Diboride-Based Ultra High Temperature Ceramics At 1500˚C. 2015.
(2) Alde, A. C. O. S. T. E. C.; Haillan, L. A Theoretical and Experimental Approach to the Active-to-Passive Transition in the Oxidation of Silicon Carbide Experiments at High Temperatures and Low Total Pressures. 3.
(3) Ghoshal, A.; Walock, M. J.; Nieto, A.; Murugan, M.; Hofmeister-, C. Gt2021- 60384. 2021, 1–12.
(4) Kane, K. A.; Pint, B. A.; Mitchell, D.; Haynes, J. A. Oxidation of Ultrahigh Temperature Ceramics: Kinetics, Mechanisms, and Applications. J. Eur. Ceram. Soc. 2021, 41 (13), 6130–6150. https://doi.org/10.1016/j.jeurceramsoc.2021.05.055.
(5) Bertin, J. J.; Cummings, R. M. Fifty Years of Hypersonics : Where We ’ ve Been , Where We ’ Re Going. 2003, 39, 511–536. https://doi.org/10.1016/S0376-0421(03)00079-4.
(6) Samvedi, V.; Tomar, V. An Ab Initio Study of ZrB 2 – SiC Interface Strength as a Function of Temperature : Correlating Phononic and Electronic Thermal Contributions. J. Eur. Ceram. Soc. 2013, 33 (3), 615–625. https://doi.org/10.1016/j.jeurceramsoc.2012.10.001.
(7) R. F. Voitovich, E. P. High-Temperature Oxidation of ZrC and HfC. 2000, 916–921.
(8) Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics; 1995; Vol. 117.
(9) Weinbub, J.; Selberherr, S. ReaxFF Reactive Molecular Dynamics Study of Orientation Dependence of Initial Silicon Carbide Oxidation. 2017, 8791–8798. https://doi.org/10.1021/acs.jpca.7b08983.
(10) Rappé, A. K.; Goddard, W. A. Charge Equilibration for Molecular Dynamics Simulations. J. Phys. Chem. 1991, 95 (8), 3358–3363. https://doi.org/10.1021/j100161a070.
(11) Grønbech-Jensen, N. Complete Set of Stochastic Verlet-Type Thermostats for Correct Langevin Simulations. Mol. Phys. 2020, 118 (8), 1662506. https://doi.org/10.1080/00268976.2019.1662506.
(12) Dünweg, B.; Paul, W. Brownian Dynamics Simulations Without Gaussian Random Numbers. Int. J. Mod. Phys. C 1991, 02 (03), 817–827. https://doi.org/10.1142/s0129183191001037.
(13) Stukowski, A. Visualization and Analysis of Atomistic Simulation Data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18 (1). https://doi.org/10.1088/0965-0393/18/1/015012.
Author Biographies
Dr. Robert Slapikas is a postdoctoral researcher at the DEVCOM Army Research Laboratory. Where previously he earned a B.S. in mechanical engineering from the Florida State University, a M.S. in materials science and engineering from the University of Florida and a Ph.D. in materials science and engineering from the Pennsylvania State University. During his time at PSU his first research steps were using molecular dynamics simulations to study electrochemical reactions at the solid liquid interface. He then worked on the oxidation reactions of ultra-high temperature ceramics through experimental static oxidation tests and reactive molecular dynamics simulations. In 2021 he was the recipient of the Oakridge Associated Universities Journeyman Fellowship and participated in the DoD High Performance Computing Internship Program in the summers of 2021 and 2022.
Dr. Anindya Ghoshal is currently a Lead Aerospace Engineer within Vehicle Power and Propulsion Branch at DEVCOM Army Research Laboratory. He leads ARL’s Turbine Power Research Team. Dr. Ghoshal is the founding member of the propulsion materials and turbomachinery sciences research area at DEVCOM Army Research Laboratory, Aberdeen Proving Ground, Maryland. He is an internationally known subject matter expert with 32+ years of research experience in Aerospace Engineering, Propulsion Materials, Turbomachinery Sciences, and Vehicle Health Monitoring Systems. After successive stints at academia, aerospace industry and NASA, he joined ARL in 2010 as the Prognostics and Diagnostics Team Lead. In the last three decades Dr. Ghoshal had participated and led several fixed wing aircraft and vertical lift rotorcraft programs both as part of industry and Government. He continues to conduct research in Turbine Engine Propulsion, Hypersonic systems, and turbomachinery sciences and serves on review panels of various Propulsion related research and development programs.
Dr. Ghoshal is a founding Associate Editor of Structural Health Monitoring Journal and an Associate Editor for Shock and Vibration Journal. Dr. Ghoshal is an AIAA Associate Fellow and the recipient of the National Research Council/NASA Langley Research Associateship, Humboldt Fellowship, Pratt & Whitney Leadership Award and Eagle Awards, Army Research Laboratory Lab Operation Award, AHS 66th Forum Structures and Materials Best Technical Paper Award (2010), AHS 71st Forum Propulsion Best Technical Paper Award (2015), AHS 73rd Systems and Reliability Best Technical Paper Award (2017), Department of Defense Laboratory University Collaborative Initiative (LUCI) Fellowship Award (2016-2019) and NATO AVT Panel Excellence Award (2022). He was inducted to the University of Connecticut’s Academy of Distinguished Engineers in 2014. Dr. Ghoshal has authored over 315+ technical publications and holds five patents. Education: M.S., PhD (UConn), MBA (RPI), MS (IIT), BS (IIEST)
Dr. Bravo is an Aerospace Engineer at the Army Research Directorate of the US Army Research Laboratory. His work involves the development of high fidelity physics based models enabling detailed investigations of complex aero-engine processes including spray breakup, chemically reacting flows, and particle-laden turbulence relevant to Army Aviation and Hypersonics S&T. He leads several research efforts in partnership with academia, industry, and cross-service agencies that span basic and early applied research in propulsion sciences. He is an Associate Fellow of the American Institute of Aeronautics and Astronautics (AIAA), holds an appointment as an adjunct Professor of Aerospace Engineering at the University of Cincinnati, University of Maryland College Park, as well as Chair of Modeling & Simulation at the Propulsion and Power System Alliance. At ARL, he also leads a DoD Workforce Development Program focused on mentoring STEM candidates in computational physics and supercomputing to address Army critical challenges. He is the recipient of multiple prestigious awards including the HPCMP Frontier, OSD SERDP, OUSD ARAP and the Army Modeling & Simulation Award.
Dr. Bravo received his PhD degree in Mechanical Engineering from the University of Maryland, College Park in 2013. Subsequently, he worked at ARL first as a post-doctoral research fellow and currently as a senior technical researcher. He has authored over 100+ research articles, delivered 30+ talks, including 2 plenary talks, and 1 patent.
Dr. Ryan McGowan is an Aerospace Engineer at the DEVCOM Army Research Laboratory (ARL). He received his BS in Aerospace Engineering from the Georgia Institute of Technology in 2012, his MS in Aerospace Engineering from the University of Notre Dame in 2016, and his PhD in Aerospace Engineering from Notre Dame in 2018. As part of the Turbine Power Research Team at ARL, Dr. McGowan focuses on experiments and modeling tools to understand the fluid and material physics that impact the performance of turbomachinery and high-speed aircraft. Dr. McGowan’s other research interests include experimental diagnostics, flow control, and alternative energy. In his free time, he enjoys outdoor activities including mountain biking, rock climbing, and skiing.
Dr. Muthuvel Murugan is a Research Aerospace Engineer in the Turbine Power Team of Mechanical Sciences Division, Army Research Directorate at DEVCOM Army Research Laboratory. He has about 14 years of research experience at ARL in a wide range of research areas such as gas turbine thermal barrier coatings, hypersonic morphing propulsion concepts, and advanced gas turbine concepts. Prior to ARL, Dr. Murugan worked in automotive industry for about 14 years and in helicopter industry for about 4 years. Dr. Murugan is an Associate Fellow of the American Institute of Aeronautics and Astronautics (AIAA) and has about 100+ publications including an US patent on adaptive turbine blade concept. He has also received several “Certificate of Achievement” awards from the Department of Army for his contributions to Army mission research. Dr. Murugan received his PhD in Aerospace Propulsion from the University of Cincinnati, Ohio and his master’s in turbomachinery from the Indian Institute of Technology, Madras, India.
Dr. Douglas E. Wolfe is currently a Professor of Materials Science and Engineering, Professor of Engineering Science and Mechanics, Professor of Nuclear Engineering, Professor of Additive Manufacturing and Design, and the Metals, Ceramics and Coatings Processing Department Head for the Applied Research Laboratory at The Pennsylvania State University. Dr. Wolfe received his BS, MS, and Ph.D. from The Pennsylvania State University in 1994, 1996, and 2001, respectively. Dr. Wolfe has over 190 peer reviewed journal articles/technical memorandums/reports, 10 patents/patents pending, and is a member of several professional societies. Professor Wolfe is a recognized international expert in the field of materials science whose research activities include the synthesis, processing, and characterization of nano, multilayered, nanostructured, functionally graded, ceramic, and metallic coatings, materials and systems deposited by reactive and ion beam assisted, electron beam physical vapor deposition (EB-PVD), cold spray, thermal spray technologies, chemical vapor deposition (CVD), cathodic arc physical vapor deposition, sputtering (r.f, d.c., magnetron), plating (Ni, Cu, Pt), hybrid processes, and various other PVD processes.

- Join us on LinkedIn to stay updated with the latest industry insights, valuable content, and professional networking!


